Nové farmaceutické a biofarmaceutické aplikace Thermo Scientific
- Foto: Pixabay/HeungSoon: Nové farmaceutické a biofarmaceutické aplikace Thermo Scientific
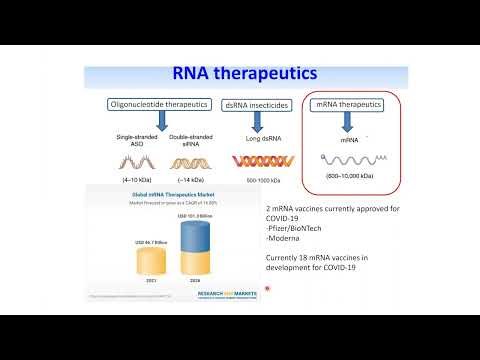
- Video: Chromatography & Mass Spectrometry Solutions: Characterization and Sequence Mapping of Large RNA and mRNA Therapeutics Using Mass Spectrometry
Přehled literatury s využitím Iontové chromatografie (5)
HPAE-PAD method for determination of Hib capsular polysaccharide content (Aplikace | 2022)
Determination of KDO from bacterial lipopolysaccharides (Aplikace | 2022)
Determination of phosphite and phosphate in ibandronate sodium (Aplikace | 2022)
Assay of guanidine in pharmaceutical formulations (Aplikace | 2022)
HPLC: Assay of Tromethamine in Pharmaceutical Formulations (Postery | 2022)
Přehled literatury s využitím Kapalinové chromatografie (10)
Simultaneous reversed-phase and anion-exchange method scouting with a dual system for mRNA impurity determination (Aplikace | 2022)
Automated UHPLC method development for mebendazole and related impurities, from method scouting to robustness testing (Aplikace | 2022)
HPLC: Automated HPLC method development and robustness tests for abacavir, lamivudine, dolutegravir and their related compounds in Triumeq drug produ (Postery | 2022)
HPLC: Simultaneous reversed-phase and anion-exchange method scouting with a dual system for mRNA impurity determination (Postery | 2022)
HPLC: Combining mass spectra, retention time modelling, and charged aerosol detection for unambiguous peak annotation and uniform-response quantitation in polysorbate profiling (Postery | 2022)
HPLC: Automated UHPLC method development and robustness test for mebendazole and related impurities (Postery | 2022)
HPLC: High-throughput analysis of oligonucleotides using a single quadrupole mass spectrometer for quality control (Postery | 2022)
Simultaneous analysis of drug substances according to USP assay and impurity methods (Aplikace | 2022)
Parallel analysis of drug product for assay and related substances determination (Aplikace | 2022)
Assessing key attributes of adeno-associated viral proteins using HPLC-FLD-intact mass analysis (Aplikace | 2022)
Přehled literatury s využitím Hmotnostní spektrometrie s jednoduchým kvadrupólem (2)
HPLC: Combining mass spectra, retention time modelling, and charged aerosol detection for unambiguous peak annotation and uniform-response quantitation in polysorbate profiling (Postery | 2022)
HPLC: High-throughput analysis of oligonucleotides using a single quadrupole mass spectrometer for quality control (Postery | 2022)
Přehled literatury s využitím Hmotnostní spektrometrie s vysokým rozlišením Orbitrap (15)
Thermo Scientific Direct Mass Technology mode (Brožury a specifikace | 2022)
HPLC: QA/QC Analytical Methods for the Structural Analysis of Biotherapeutic Viruses (Postery | 2022)
ASMS: Retention Time Based FAIMS Depletion of Protein Drugs in Peptide Mapping to Increase Coverage of Background Host Cell Proteins (Postery | 2022)
ASMS: Characterization and quantification of lipid nanoparticle components and their degradants in vivo using an LC-HRAM MS platform (Postery | 2022)
ASMS: Characterization of monoclonal antibodies and antibody drug conjugates using iCIEF-MS online coupling platform (Postery | 2022)
ASMS: Characterization of mRNA vaccine 5’ and 3’ end products using BioPharma Finder 5.0 (Postery | 2022)
ASMS: Accelerated Solvent Extraction & Ultra-High Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry For Analysis of Additives in Polymers For Biomanufacturing Processes (Postery | 2022)
ASMS: Streamlining workflow from characterization to monitoring of therapeutic oligonucleotides impurities across IPRP-LC-HRAM-MS platforms (Postery | 2022)
The MAM 2.0 workflow enables seamless transition from research and development to quality control (Aplikace | 2022)
Assessing key attributes of adeno-associated viral proteins using HPLC-FLD-intact mass analysis (Aplikace | 2022)
Consistent results for peptide mapping and monitoring across three systems of the Vanquish UHPLC platform (Aplikace | 2022)
mRNA direct sequence mapping using automated partial digestion with magnetic nuclease and LC-HRMS (Aplikace | 2022)
Characterization of in vitro-transcribed (IVT) mRNA poly(A) tail by LC-HRAM-MS and BioPharma Finder 5.0 software (Aplikace | 2022)
Characterization of mRNA 5’ capping products using an LC-HRAM-MS/MS analytical platform and Thermo Scientific BioPharma Finder software solution (Aplikace | 2022)
A validated LC-HRAM-MS method for the rapid and confident determination of azido (AZBT) impurity in sartan drug products (Aplikace | 2022)
Pro více informací nás neváhejte kontaktovat na [email protected]




